BEE VENOM TREATMENT AND STUDIES FOR ALS
|
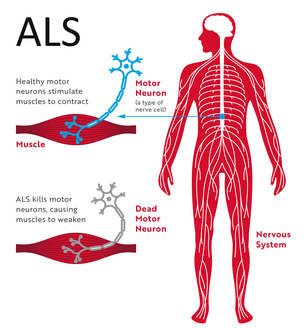
What is ALS?
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that leads to the death of neurons in the brain stem, brain, and the spinal cord. It ultimately causes paralysis and loss of muscle mass. In several cases, it has been reported that this disease spreads to legs, arms and even the tongue. Researchers have found different theories that help explain the reasons behind the manifestation of ALS. It is suggested that structural changes of protein can lead to malfunctioning and death of nerve cells. Considerable research has been done in the field of ALS over the past 10 years. One of the most prominent is the European project that consists of 15,000 subjects of ALS. A research group from Umea University, having 500 Swedish ALS patients, will also become a part of this extensive project. |
Photo: ALS.org
The most common type of ALS consists of, SOD1, a misfolded protein that accumulates within neuron transmitters. Researchers are focusing on the reasons behind this misfolding and how it spreads and affects the nervous system. The main focus of various research groups is to devise methods that can help deter the formation and production of SOD1. It is to be noted that a few types of ALS and FTD (boiler lobe dementia) nearly have the same disease process that affects different parts of the nervous system. According to the researchers – it is due to the reason that both diseases have the same genes. It still needs to be assessed that why some patients become a victim of FTD and others ALS.
Melittin Restores Proteasome Function In An Animal Model Of ALS
Amyotrophic lateral sclerosis, is also known as ALS, is a chronic paralyzing disorder that is caused due to deterioration of motor neurons/ it can occur both as a familial and sporadic disease. MtSOD1 (mutant SOD1) present in motor neurons become vulnerable to this disease due to protein misfolding, neuroinflammation, excitotoxicity, defective growth factor signaling and axonal transport, cytoskeletal abnormalities, oxidative damage, and mitochondrial dysfunction.
Bee venom is loaded with essential medicinal ingredients. Melittin is one of those elements that are found in bee venom. It is a 26 amino acid chain, which has been used since long in various traditional Chinese medicines to get relief from pain and inflammation. The main purpose of this study was to assess the medicinal benefits of melittin in subjects of ALS. The commonly used hSOD1G93A mouse was chosen for this clinical study that had inherited ALS. Melittin was given at ST36 acupuncture point to determine if it could help prevent protein misfolding and suppress neuron loss.
It was found that the hSOD1G93A mouse treated with melittin showed a definite decrease in the count of microglia and phospho-p38 in the brain stream and spinal cord. Melittin also helped in improving the condition of neurons in the spinal cord and overall motor function compared to the control group. A prominent increase of α-synuclein modification like nitration and phosphorylation was also observed in the spinal cord and main brainstem of hSOD1G93A mouse.
Finally, it can be said that melittin administration reduced the misfolding of α-synuclein and restored proteasomal activity in the spinal cord and brainstem of symptomatic mice. Research suggests that there exists a potential link between melittin administration and retrogression of neuro-inflammation in the ALS hSOD1G93A mouse models.
Bee Venom Attenuates Neuroinflammatory Events and Extends Survival
ALS stands for amyotrophic lateral sclerosis. It is a disease that affects the central nervous system and causes various disorders. Death of motor neurons is the main cause of this propagative disease – it can be due to familial origin or sporadic. The genetic factor that contributes towards the appearance of ALS is the mutant SOD1 that induces the vulnerability of the motor neurons due to protein misfolding, neuroinflammation, glutamate excitotoxicity, defective axonal transport, cytoskeletal abnormalities, oxidative damage, and mitochondrial dysfunction.
BV (bee venom) has been used for a long time in oriental medicine. Proper literary evidence suggests that bee venom has anti-inflammatory properties against inflammatory reactions that are associated with various inflammatory diseases like arthritis. The main point of the study conducted was to analyze the effect of bee venom on hSOD1G93A mutant mice that whether BV helps in suppressing the loss of motor neurons or not.
BV (bee venom) was bilaterally administered at ST36 acupoint into two weeks old hSOD1G93A male mice. Zusanli (ST36) acupoint is chosen because it is known to mediate the anti-inflammatory effect. To check the motor activity, a special analysis called the rotarod test was carried out. Survival statistics were investigated using the Kaplan- Meier survival curve method. The overall effect of bee venom treatment on the anti-neuroinflammation of the hSOD1G93A model male mice was assessed through immunoreactions by using TNF – α antibody and Iba 1 as microglia marker. Activation of caspase 3 proteins, p 38 MAP Kinase and ERK were weighed by using western blotting.
HSOD1G93A transgenic mice, which were treated with bee venom showed a significant decrease in the level of phosphor-p38 MAPK and microglia marker in the brainstem and spinal cord. Moreover, the administration of bee venom in the symptomatic ALS subjects helped in improving overall motor activity. The median survival, compared with the control group, of the bee venom treated animals was found to be 18% greater. It was also found that bee venom helped in blocking flaws of cristae morphology and mitochondrial structure and suppressing levels of caspase-3 activity in the spinal cord of ALS mice.
In light of this clinical study, the research suggests that bee venom can be used as an effective therapeutic medicine for addressing ALS effects in hSOD1G93A mutant mice.
Melittin Restores Proteasome Function In An Animal Model Of ALS
ALS is one of the most rapidly progressing neurodegenerative diseases, which is caused due to the death of neurons in upper motor neurons and spinal cord. As a result, it leads to paralysis of the attached muscles. Transmutations in the SOD1 (Zn/Cu superoxide dismutase), account for nearly 20% of the inherited cases of FALS (ALS). The transgenic hSOD1G93A mice develop cardinal symptoms of Amyotrophic lateral sclerosis in humans that include muscle atrophy and paralysis. The pathophysiological mechanisms of Amyotrophic lateral sclerosis include inflammation, disrupted axonal transport, glutamate excitotoxicity, and mitochondrial dysfunction.
Bee venom is extracted directly from the honey bees and its generic name is apitoxin. Traditionally, it has been used to control inflammation in the subjects of osteoarthritis and chronic rheumatoid arthritis. It has been already established, in previous investigations, that bee venom possesses anti-neuroinflammatory traits. Bee venom has a good number of peptides like apamin and melittin and other bioactive compounds. Melittin is a powerful amino acid polypeptide that makes up to nearly 60% of the dry honey bee venom. It is also believed to be the foremost biologically active substance, found in bee venom, which generates anti-inflammatory and anti-nociceptive effects when registered to an acupoint.
HSOD1G93A mice were used for the lab study by the guidelines set forward by the national institute of health (Bethesda, MD). Essential protocols were agreed by Use Committees of the Korea Institute of Oriental Medicine and Institutional Animal Care. Transgenic B6SJL mice were obtained from Jackson Laboratory – bearing glycine to alanine modification at 93rd codon of cytosolic Zn/CU superoxide dismutase genetic type (HSOD1G93A). All of the subjects (mice0 were provided ample access to water, rodent chow, and standard housing.
For treatment reasons, Melittin was obtained from the Sigma St. Louis, MO, and then later diluted with some saline. 98 weeks old male mice were injected bilaterally with melittin at the ST36 acupoint. This point is known to deliver anti-inflammatory effects. Male mice were given melittin two times a week. The age-matched animals of the control group were injected bilaterally with equal volumes of saline at the same acupoint.
98 weeks old mice were later divided into two different groups: the saline-treated and the melittin treated. The lifespan of the treated subjects was measured with the help of Kaplan Meier survival curves using Sigmaplot 10 software and Prism 5.0 software. The values obtained were carefully analyzed by one way ANOVA and Dunn’s multiple comparison tests.
It was found that a proper regime of melittin helped increase motor performance in the hSOD1G93A transgenic mice. According to the results of the rotarod behavioral test, the motor functioning increased by 1.7 folds after 7 days of treating hSOD1G93A mice. HSOD1G93A mice treated for 10 days with melittin showed an impressive 2.8 fold improvement in the motor activity.
It was also found that paralysis and disease onset was delayed by 1 week in those mice, which were treated by melittin compared to those that were only given saline. However, it was found that melittin treatment does not affect the survival rate or span of the mice, regardless of the methods that they were studied in the lab. The median survival of the control group was somewhat close to the melittin treated group – 129 and 132 days respectively. One of the most notable developments was that the melittin treated mice displayed a delayed onset of the disease when compared with the age-matched saline-treated mice.
Hence, the results depict that melittin treatment over a particular period helps delay the progression of the disease and the inception of motor dysfunction but doesn't contribute towards extending the lifespan of selected mice.
The lab study reveals that melittin significantly reduces neuro-inflammatory developments and helps decrease a-synuclein modification by increasing proteasome activity in the mice. It suggests that chaperone proteins and proteasome activity are both responsible for clearing any misfolded proteins like synuclein and SOD1.
Adding Furthermore, the link between PD and ALS remains unclear. Additional experiments and further studies in the light of them are required to establish the mechanism that PD and ALS share. It will also help in clarifying the link between protein and melittin treatment.
Effects of Bee Venom on Glutamate-Induced Toxicity in Neuronal and Glial Cells
Glutamate plays a vital role in regulating brain functions like cognition, learning, synaptic plasticity, and memory. When the glutamate receptors are disturbed it can lead to excitotoxicity. Overstimulation of the NMDA receptors through glutamate can lead to abnormal neuronal degeneration followed by seizures, hypoglycemia, ischemia, and hypoxia. Glutamate neurotoxicity is associated with different neurodegenerative disorders like Alzheimer's disease.
Motor neuron death can be linked with several toxic factors that have the possibility of arising from different types of cells. When patients of ALS are analyzed it is found that they have altered levels of glutamine synthetase, decrease the level of EAAT2, decreased glutamate uptake and increased levels of plasma related glutamate. Bee venom has been used in this study for the pretreatment of microglial and neuronal cells. Results derived might have any clinical implications and that BV can be used as a potential treatment for preventing neurogenerative and inflammatory diseases.
Bee venom, tween-20, L-glutamate, 5-diphenyltetrazolium bromide and 3-(4, 5 dimethylthiazol-2-yl) were obtained from Sigma, USA. While the lactate dehydrogenase kit was obtained from the Biovision Research Products, USA and the Fetal Bovine serum from Gibco. Primary antibodies required for p38, p-p38, ERK, p-ERK, AKT, JNK, p-JNK were attained from Cell Signaling Technology, USA.
Cell membrane cytotoxicity was examined using the LDH release assay that quantitatively processes activity of LDH - a stable enzyme that is released when a cell receives some sort of damage. To carry out this study microglial and neuronal cells were plated on 96 well plates. Later, these cells were subjected to a controlled concentration of bee venom for 30 minutes before getting the glutamate treatment for 2 days. After that culture supernatants were collected from the 96 well plates and then evaluated for LDH release by the instructions put forward.
A microplate reader was used to measure absorbance and after-effects of a 10-minute reaction. Mean change linked with absorbance was examined for each treatment. It is expressed as a percentage of absorbance of the control cell supernatants.
Total cellular proteins were subjected to isolation with the help of an ice-cold lysis method containing 50mM Tris having HCl with pH 7.4. The concentration of protein was established with the BCA Protein Assay Kit.
It can be said that this study is one of the most useful bases for determining that bee venom prevents cell death and activates pro-apoptotic signaling in the glutamate stimulated cells. Bee venom also offsets cell toxicity by inhibiting the p38 and JNK pathways. All of these findings certainly put special emphasis on the medicinal importance of bee venom for treating inflammatory and glutamate-mediated syndromes.
Sources:
Melittin restores proteasome function in an animal model of ALS.
Bee venom attenuates neuroinflammatory events and extends survival.
Melittin restores proteasome function in an animal model of ALS.
Effects of Bee Venom on Glutamate-Induced Toxicity in Neuronal and Glial Cells.
Melittin restores proteasome function in an animal model of ALS
Melittin restores proteasome function in an animal model of ALS.
Bee venom attenuates neuroinflammatory events and extends survival.
Melittin restores proteasome function in an animal model of ALS.
Effects of Bee Venom on Glutamate-Induced Toxicity in Neuronal and Glial Cells.
Melittin restores proteasome function in an animal model of ALS